Abstract
Introduction: Chimeric antigen receptor (CAR) T cell therapy targeting CD19 has recently demonstrated high success but also shown limitations regarding their toxicity and development of CD19negative variants. Here we reported results from a phase I study designed to determine the safety of the CD19 CAR-T and CD22 CAR-T cocktail and the feasibility of making enough quantities to treat patients with CD19+CD22+ relapsed/refractory B cell acute lymphoblastic leukemia (B-ALL).
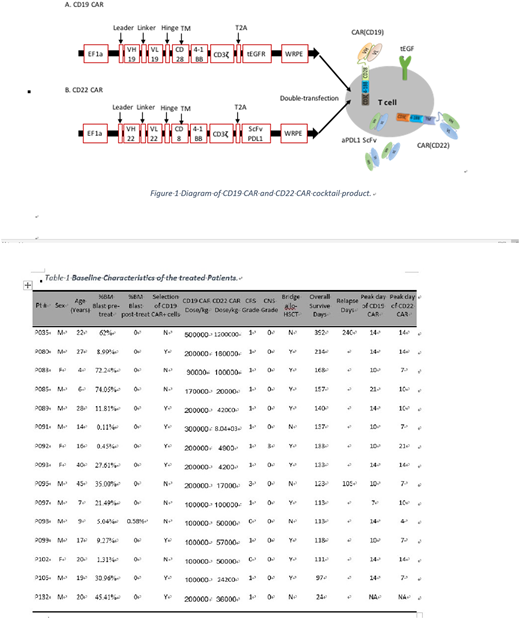
Patients and Methods: From July 2017 to July 2018, a total of 15 patients with CD19+CD22+ relapsed/refractory B-ALL were treated, including 5 children and 10 adults (Table 1). All patients received fludarabine 30mg/m2/d´3d and cyclophosphamide 250mg/m2/d´2d before infusion of CAR-T cells, followed by a cocktail CAR-T cell infusion with a median number of 2 (0.9-5)´105 CD19 CAR+ T cells/kg and a median number of 0.5 (0.4-12)´105 CD22 CAR+ T cells/kg. The lentiviral backbone containing constructs of CD19 CAR and CD22 CAR are shown in Figure 1. CD19 CAR includes a truncated EGFR sequence which can be used to identify and select CAR+ cells. CD22 CAR includes a single chain variable fragment (ScFv) sequence derived from a monoclonal antibody against human PD-L1 which attempts to reduce the exhaustion of CAR-T cells by blocking the PD-1/PD-L1. Real-time quantitative PCR using primers with specificity for the ScFv of CD19 CAR and ScFv of CD22 CAR can detect the in vivo CAR-T persistence for either CAR. Sequential transduction was performed 2 days after activation of sorted T cells stimulated with CD3 and CD28 antibodies. Percentages of CD19 CAR+ and CD22 CAR+ T cells were determined by flow cytometry through staining with an antibody against EGFR and a fusion protein of CD22-Fc, respectively, and expression of anti-PDL1 ScFv in CD22 CAR-T cells were demonstrated by flow cytometry through intracellular staining with a PD-L1-Fc fusion protein. The primary end points of this study were to evaluate feasibility and toxicity, and the secondary end points included disease response and persistent CAR-T engraftment of infused CAR-T cell.
Results: The median observation period was 133 days (24-392 days). The median percentage of pre-treatment bone marrow CD19+CD22+ blasts was 21.5%(0.11-74.1%). On day 20-30 after CAR-T infusion, 15/15 (100%) cases achieved complete remission (CR) or incomplete CR(CRi), 14/15 (93.3%) cases had negative minimal residual disease (MRD). Patient P098 had residual (0.58%) CD19+CD22+ BM blasts at day 30 post-infusion and thereafter achieved negative MRD after re-infusion with CD19 CAR-T cells. 11/17 patients were bridged into allo-HSCT and have remained in remission state with a median follow up of 133 (97-214) days. 2/5 patients without bridging allo-HSCT relapsed on day 240 and day 105 post-infusion, respectively. Notably, both patients (100%) relapsed with CD19+CD22+ leukemia cells. Despite achievement of a very high CR rate, a very low treatment-related toxicity was observed in this trial. Only 1 patients experienced grade 3 cytokine release syndrome (CRS) and another patient (6.7%) developed grade 3 central nervous system (CNS) toxicity; all other patients were CRS grade<2 and CNS grade 0. On days -1, 1, 4, 7, 10, 14, 21 and 28 after infusion, peripheral blood (PB) was drawn and the level of infused CD19 and CD22 CAR-T cells were analyzed by either qPCR or flow cytometry assay. Results demonstrated obvious in vivo proliferation of both CD19 and CD22 CAR-T cells. The median peak level was 3.5 (0.47-79.1)´104 copy number/mg PB genomic DNA for CD19 CAR-T, and 0.9 (0.08-80.8)´104 copy number/mg PB genomic DNA for CD22 CAR-T. The median day to reach the peak value was day 10 for both CARs, ranging mostly from day 7 to day 14.
Conclusion: This study demonstrates technical feasibility, high efficacy and low toxicities of CD19 and CD22 CAR-T cocktail in treating patients with CD19+CD22+ relapsed/refractory B-ALL. Both patients relapsed with CD19+ leukemia suggests this cocktail treatment may reduce the risk of CD19 negative relapse. Low toxicities may relate with small number of infused CAR-T, but involvement of anti-PDL1 ScFv which is co-expressed with CD22 CAR construct cannot be excluded. Therefore, related mechanisms are currently being investigated in the lab.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal